RESERCH
■EDEM; ER-degradation enhancing alpha-mannosidase like protein
We are working on the molecular mechanism of ERQC (ER quality control) and ERAD (ER-associated degradation) in mammals.
Many works have clarified the importance of ERAD of misfolded proteins and the disruption of ERQC in genetic diseases and neurodegenerative disorders.
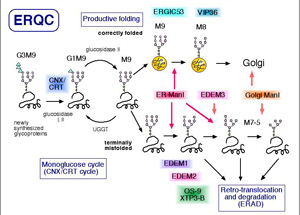
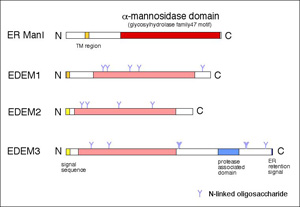
We have previously cloned a mouse gene EDEM (ER degradation enhancing -mannosidase-like protein), which is involved in the ERAD of glycoproteins.
There are three EDEM homolog proteins in mammals and one paralog in yeast.
Although all of the homologs enhance glycoprotein ERAD, the molecular mechanism whereby each protein works seems to be different.
We are now investigating the functional divergence as well as the comprehensive mechanism of these EDEM family proteins.
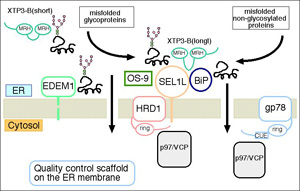
We are also working on the involvement of N-linked sugars in the ERQC, the molecular mechanism of retrotranslocation of misfolded substrates, and the molecules responsible for the ubiquitination of ERAD substrates on the ER membrane.
Recently, we have found that two novel mammalian lectins XTP3-B and OS-9 make a complex with a membrane-embedded ubiquitin ligase HRD1, forming an ER quality-control scaffold.
Biochemical analysis of the oligosaccharide structures that these ER lectins recognize has further clarified the mechanism how glycoprotein ERAD is regulated through the processing and recognition of the N-glycans.